Every chemical process involves the transfer of energy. Here, we will learn about 2 important chemical processes: exothermic and endothermic reactions, how they differ, and why they are so important when it comes to deicing roadways.
The Movement of Heat
The laws of thermodynamics state that energy cannot be created or destroyed. Instead, energy is transferred and changes forms constantly all around us. Some reactions need an input of energy, and some give off energy. This movement of energy can take different forms, such as light or heat.
Endothermic Reactions
Endothermic reactions require an input of energy (in the form of heat) in order for the reaction to start. This input of heat energy from the surroundings, triggers a breakdown of both chemical and physical bonds.
One of the most common endothermic reactions is the melting of ice and snow. Heat is drawn in from the surroundings, triggers this reaction, and the chemical and physical bonds holding the ice together begin to break.

Exothermic Reactions
Exothermic reactions release energy (in the form of heat) out into their surroundings. Examples of exothermic reactions include campfires, lighting matches, etc.

Did you know, water freezing into ice is also exothermic? Heat has to leave the water, and release into its surroundings in order for it to become cold enough for ice to form.
The Thermodynamics of Winter Road Maintenance
Continuous freezing, melting, and refreezing cycles wreck havoc on our roads during the winter. All winter road maintenance programs try to encourage the endothermic reaction of ice melting, and interfere with the reaction of ice freezing.
Ice Adhesion
When water freezes into ice, its molecules interlock with each other and create hydrogen bonded, crystalline structures. Ice can bond, or adhere, to surfaces physically, chemically, and even electro-magnetically.
This adhesion can be difficult to break, especially on rough road surfaces. Road safety agencies spend time and resources researching how to break ice’s bond on our roadways. The best road maintenance practices use a combination of chemical and physical methods to break this icy adhesion.
Physical:
- Build strategic snow fences to prevent drifts from forming on the roadway
- Mechanical ice and snow removal with snow plows
Chemical:
- Pre-storm anti-icing applications to stop ice from adhering to roads in the first place
- Deicing treatments, during and after a storm, to trigger an endothermic reaction, lower ice’s melting point, and delay or stop refreezing cycles
Not all chemical deicing treatments are created equal. Some products trigger more powerful endothermic reactions and work better at lower temperatures. Click here to learn more about how different deicing ingredients work and can vary in strength.
Ice Slicer: High Endothermic Performance
Harvested from a Jurassic Era mineral deposit, Ice Slicer is nature’s perfect recipe for melting ice and snow. 60+ trace minerals give Ice Slicer its signature reddish hue that helps boost its endothermic performance.
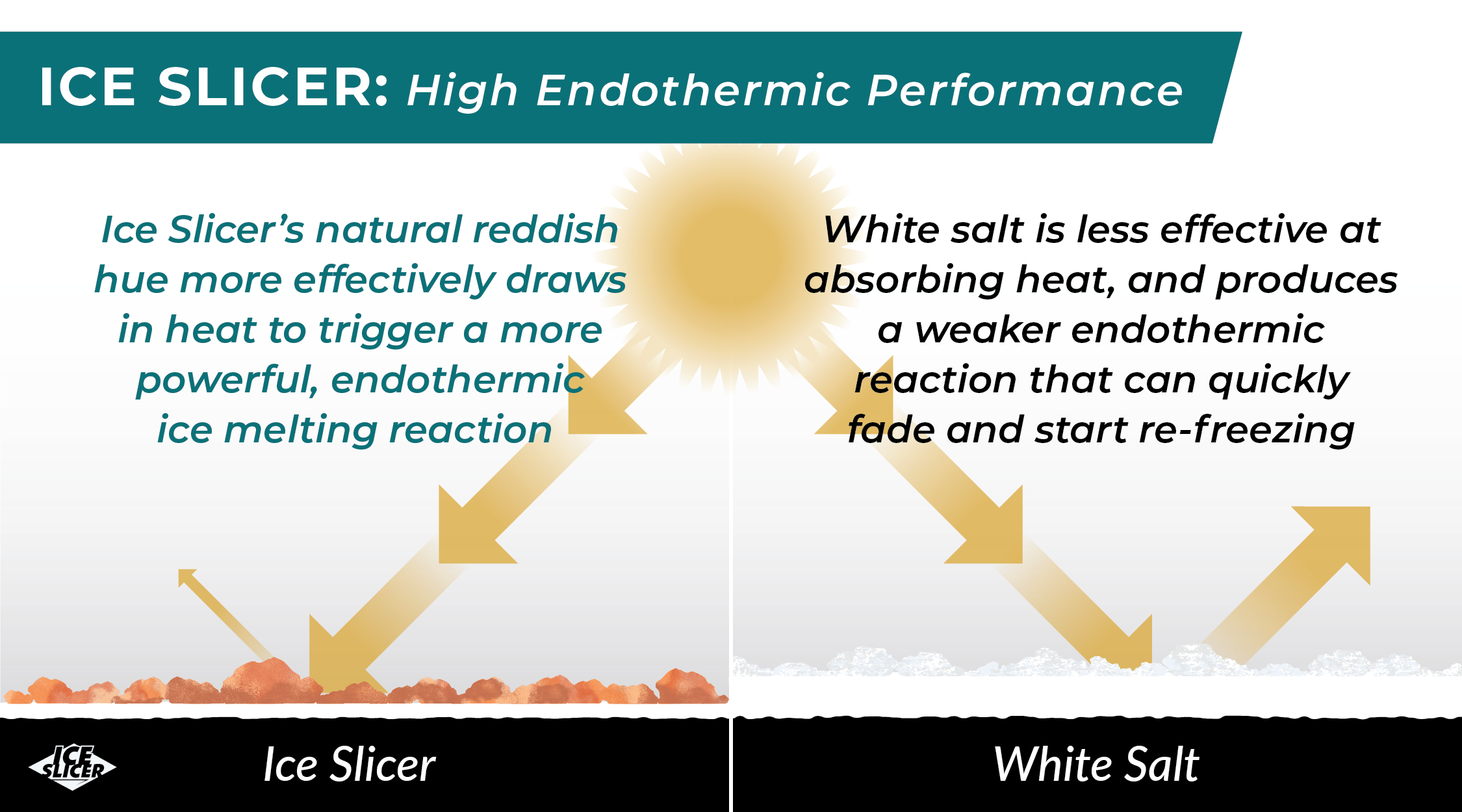
This natural, darker color lowers Ice Slicer’s albedo factor, enabling it to more efficiently capture heat energy from the sun to melt ice quickly. Other deicing treatments add artificial dyes to darken their products; however these dyes can bioaccumulate and harm our environment. Ice Slicer doesn’t need any added chemicals to naturally outperform white salt, quickly restore road safety, and keep roads clear longer.

